Background:
The Chimeric antigen receptor (CAR) T cell therapy for hematological malignancies has revolutionized anticancer therapy. CAR-T cell expansion and persistence are strikingly associated with the intrinsic programmes of T cells. However, the current process for CAR-T cells manufacturing resulted in high frequencies of exhausted and senescent CAR-T cells with reduced mitochondrial mass. T cell receptor (TCR) signaling cooperates with co-stimulatory signaling to regulate T cells activation and differentiation. Considering that ICOS has a well-defined role in T cell differentiation, especially with regard to the incorporation of ICOS into CAR to boost CAR-T cells antitumor activity, we therefore hypothesize that TCR activation in synergy with dual CD28 and ICOS co-stimulation rapidly triggers T cell activation and may be a potential approach to optimize CAR-T cells antitumor activity.
Methods: In this study, we established a culture system using plate-coated CD3 in combination with CD28 and ICOS mAbs to simultaneously stimulate T cells, and further studied whether dual CD28 and ICOS co-stimulation could reprogram the transcriptional, epigenetic and proteomic states of T cells and optimize CAR-T cells antitumor activity. In vitro CAR-T cell phenotype and cytotoxicity were detected via flow cytometry, co-culture killing assay and degranulation assay. NSG mice were inoculated with Raji cells to establish xenograft mouse model, and the tumor load and survival time were dynamically monitored after treatment with CAR-T cells.
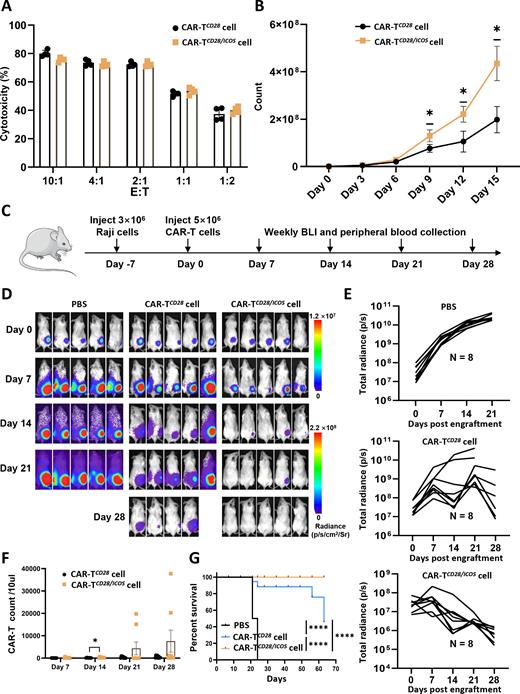
Results: As expected, dual co-stimulation potently enhanced T cells activation and expansion via reprogramming their transcriptome, epigenome and proteome. Mechanistically, dual CD28 and ICOS co-stimulation rapidly upregulated the pathways such as ribosome, citrate cycle (TCA cycle) and oxidative phosphorylation (OXPHOS) to drive T cells exiting from quiescence. Moreover, metabolic pathways are rewired in CD28 and ICOS-stimulated T cells to guarantee sufficient energy for cell proliferation. Meaningfully, dual co-stimulation confers CAR-T cells with high lentiviral transfection efficiency, possibly correlating with highly open chromatin accessibility in CD28 and ICOS-stimulated T cells. CAR-T cells manufactured with dual CD28 and ICOS co-stimulation demonstrated significantly enhanced OXPHOS and mitochondrial biogenesis, resulting in a superior expansion and being more effective at killing tumor cells in immunodeficient animals.
Conclusions: Collectively, our data indicated that activating CD28 and ICOS signaling during CAR-T cells preparation is a potential strategy for optimizing CAR-T cells antitumor activity.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal